
Yingdi Yan
Maria Curie Sklodowska University, Poland
Title: Fabrication of cell membrane mimic phospholipid polymer brush surface and its protein resisitant property
Biography
Biography: Yingdi Yan
Abstract
As a promising method to obtain well designed adaptive biointerfaces, polymer brush has aroused broad interest worldwide. Surface initiated polymerization (SIP) has been proved an effective way to obtain thick and dense polymer brush. The newly reported Cu (0) mediated controlled radical polymerization (CuCRP) showed advantanges with repect to lack of toxic halide, tolenrence to oxygen, little reaction solution consumption and reusage of copper plate as a catalyst. To facilitate the biocompatibility and suppress unfavourable protein adsorption, 2-methacryloyloxyethyl phosphorylcholine (MPC) was used, the structure of which is designed to mimic the the main component of biological cell membranes, phospholipid. This is the first time to report PMPC brush fabrication with SI-CuCRP and investigate the mechanism of protain resistance from the viewpoint of surface free enery analysis.
The polymerization process was tracked by XPS and static contact angle measurement and the surface topography was ananlyzed by optical profilometer and AFM. The growth rate of the polymer brush is extremely high and the film thickness could be adjusted by reaction variability, e.g. monomer concentration and reaction time. The graft density was calculated with the help of ellipsometry measurement in dry (0% humidity) and wet (>96%) conditions and its dependence on the monomer concentration and swelling ratio was found. The hydrophilicity was analyzed by surface free energy and its components calculation based on LWAB and CAH approaches and its relationship with protein resistant property was established. The total surface free energy was augmented compared with the untreated surface which are mainly attributed to the polar components increase because the London dispersion interactions are almost the same in spite of the polymer brush modification. An optimal thickness of PMPC implies protein resistance related to surface conformation. Probably self-condensation or charged segments being packed inside the film led to weak surface hydration.
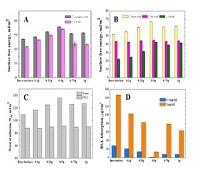
Figure: (A) Apparant total surface free energy of PMPC grafted surfaces with different monomer concentration. (B) Individule CAH surface free energy of PMPC grafted surfaces calculated from water, diiodomethane contact angles and the electron-donor parameter gs- calculated from LWAB method. (C) Work of adhesion of water and diiodomethane to PMPC grafted surfaces; (D) BSA adsorption on the bare surface and PMPC modified surface.

